

Industrial Inorganic Chemistry
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
The extraction of metals by carbon reduction, The extraction of metals by electrolysis, Ammonia and fertilisers, Sulfur and sulfuric acid, The chlor–alkali industry, Limestone, The economics of the chemical industry.
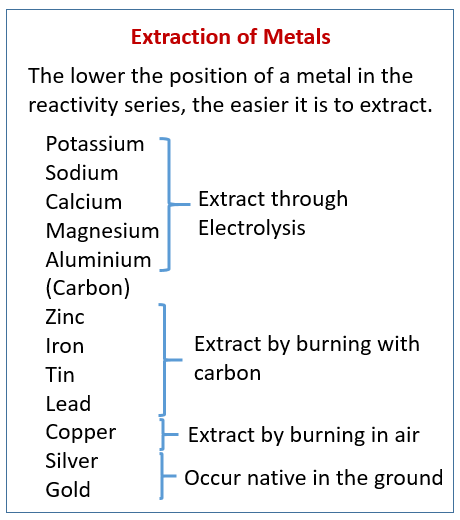
The following table shows how the method for the extraction of a metal depends on its position in the reactivity series. Scroll down the page for more examples and explanations.
The following diagram shows the steps to convert iron from the blast furnace to steel. Scroll down the page for more examples and explanations.
How we extract metals from ores. How we extract iron using carbon in a blast furnace. Alloys of iron
We can turn pure iron into the alloy steel.
We explore why steel is hard and look at low-carbon and high-carbon steel.
Finally, we take a look at stainless steel Metal reactivity - Iron, Rusting and Prevention
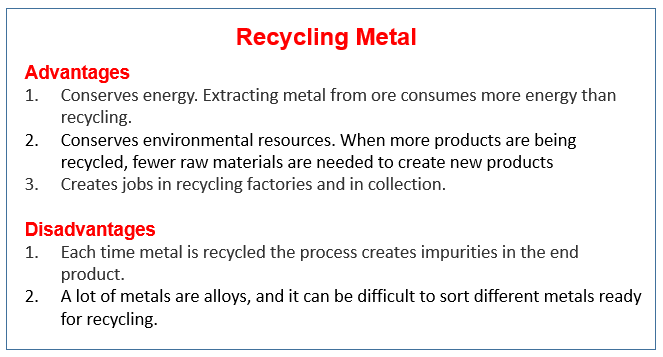
Recycling metals
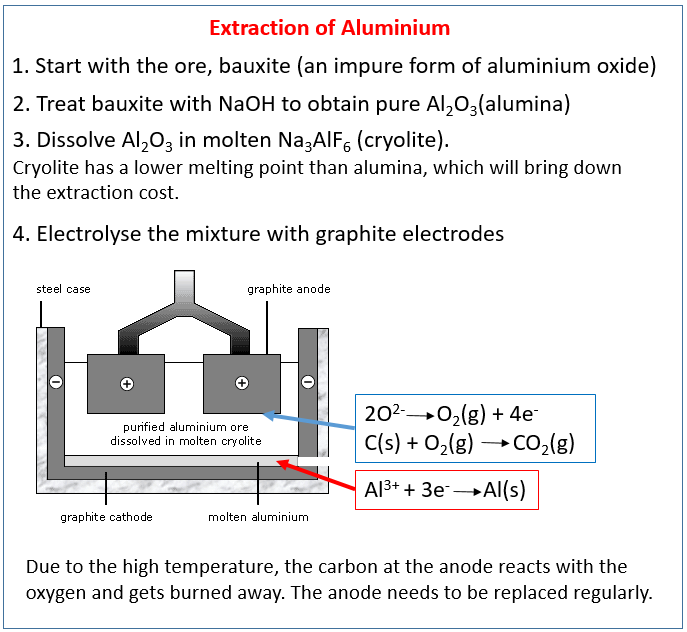
Two really important metals: titanium and aluminium.
What makes them so expensive?
Three good reasons why we need to recycle metals. Extraction and Uses of Metals
How to extract Aluminum and Iron?
Extraction of Zinc
Electrolysis
Properties of Group 1: The Alkali Metals, Group 7: The Halogens, Group 0: The Noble Gases
The vocabulary used in electrolysis and how the process works
How to work out what the products will be when doing electrolysis of ionic compounds when molten, and when in solution?
What are half-equations are also covered?
The chlor-alkali industry as a specific example of the use of electrolysis in industry. Electrolysis of brine.
Contact process
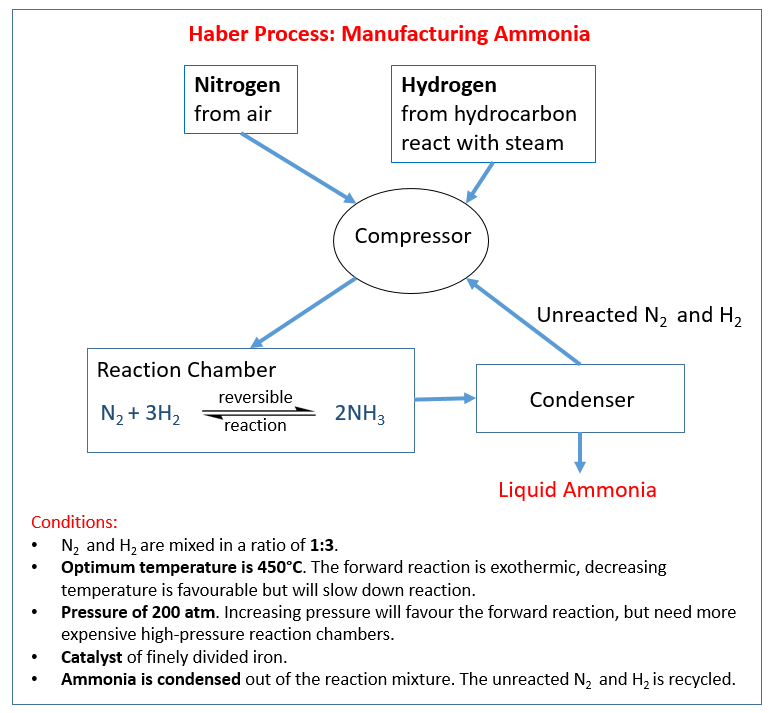
How to make sulfuric acid? Haber process
How to make ammonia? Ammonia and Fertilisers
Fertilisers contain minerals such as Nitrates, Phosphates and Potassium compounds which are needed for healthy plant growth.
Nitrogen from nitrates is needed to make amino acids for proteins.
Phosphorus from phosphates is needed to make DNA and cell membranes.
Potassium from potassium compounds is needed in enzymes involved in respiration and photosynthesis.
NPK values show the relative proportions of nitrates, phosphates and potassium in a fertiliser. They help farmers decide which fertiliser is best for the soil conditions in their fields. Equilibrium and Industrial Processes
Reversible reactions, the idea of chemical equilibrium, and how conditions affect the position of equilibrium, before considering the Haber Process (Ammonia) and the Contact Process (Sulfuric Acid).
How taking control of the equilibrium allows each of these processes to be optimised?
Chlor-Alkali Process
What is sulfur?
Sulfuric Acid properties
Limestone to make cement
Thermal decomposition of limestone
What happens to limestone when we heat it. This process is called thermal decomposition and it is a frequent exam question. Calcium hydroxide and limewater
Thermal decomposition of limestone
Thermal decomposition of metal carbonates, reactions of metal carbonates with acids, advantages and disadvantages of quarrying, the limestone cycle equations and chemical weathering.


More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
The extraction of metals by carbon reduction, The extraction of metals by electrolysis, Ammonia and fertilisers, Sulfur and sulfuric acid, The chlor–alkali industry, Limestone, The economics of the chemical industry.
The following table shows how the method for the extraction of a metal depends on its position in the reactivity series. Scroll down the page for more examples and explanations.
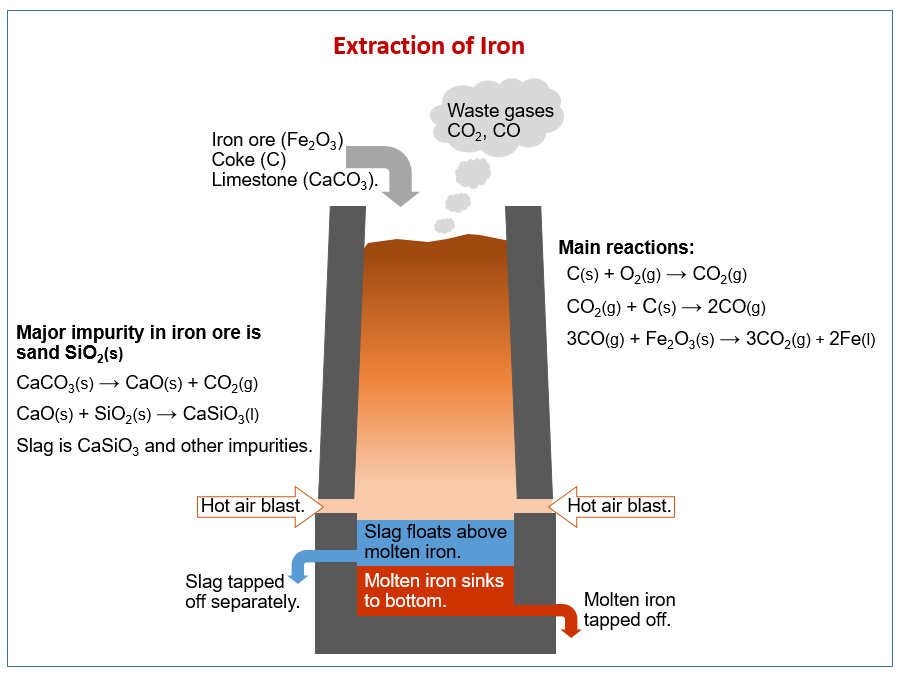

How we extract metals from ores. How we extract iron using carbon in a blast furnace. Alloys of iron
We can turn pure iron into the alloy steel.
We explore why steel is hard and look at low-carbon and high-carbon steel.
Finally, we take a look at stainless steel Metal reactivity - Iron, Rusting and Prevention
Recycling metals
Two really important metals: titanium and aluminium.
What makes them so expensive?
Three good reasons why we need to recycle metals. Extraction and Uses of Metals
How to extract Aluminum and Iron?
Extraction of Zinc
Electrolysis
Properties of Group 1: The Alkali Metals, Group 7: The Halogens, Group 0: The Noble Gases
The vocabulary used in electrolysis and how the process works
How to work out what the products will be when doing electrolysis of ionic compounds when molten, and when in solution?
What are half-equations are also covered?
The chlor-alkali industry as a specific example of the use of electrolysis in industry. Electrolysis of brine.
Contact process
How to make sulfuric acid? Haber process
How to make ammonia? Ammonia and Fertilisers
Fertilisers contain minerals such as Nitrates, Phosphates and Potassium compounds which are needed for healthy plant growth.
Nitrogen from nitrates is needed to make amino acids for proteins.
Phosphorus from phosphates is needed to make DNA and cell membranes.
Potassium from potassium compounds is needed in enzymes involved in respiration and photosynthesis.
NPK values show the relative proportions of nitrates, phosphates and potassium in a fertiliser. They help farmers decide which fertiliser is best for the soil conditions in their fields. Equilibrium and Industrial Processes
Reversible reactions, the idea of chemical equilibrium, and how conditions affect the position of equilibrium, before considering the Haber Process (Ammonia) and the Contact Process (Sulfuric Acid).
How taking control of the equilibrium allows each of these processes to be optimised?
Chlor-Alkali Process
What is sulfur?
Sulfuric Acid properties
Limestone to make cement
Thermal decomposition of limestone
What happens to limestone when we heat it. This process is called thermal decomposition and it is a frequent exam question. Calcium hydroxide and limewater
Thermal decomposition of limestone
Thermal decomposition of metal carbonates, reactions of metal carbonates with acids, advantages and disadvantages of quarrying, the limestone cycle equations and chemical weathering.
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.