

Condensation Polymers
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
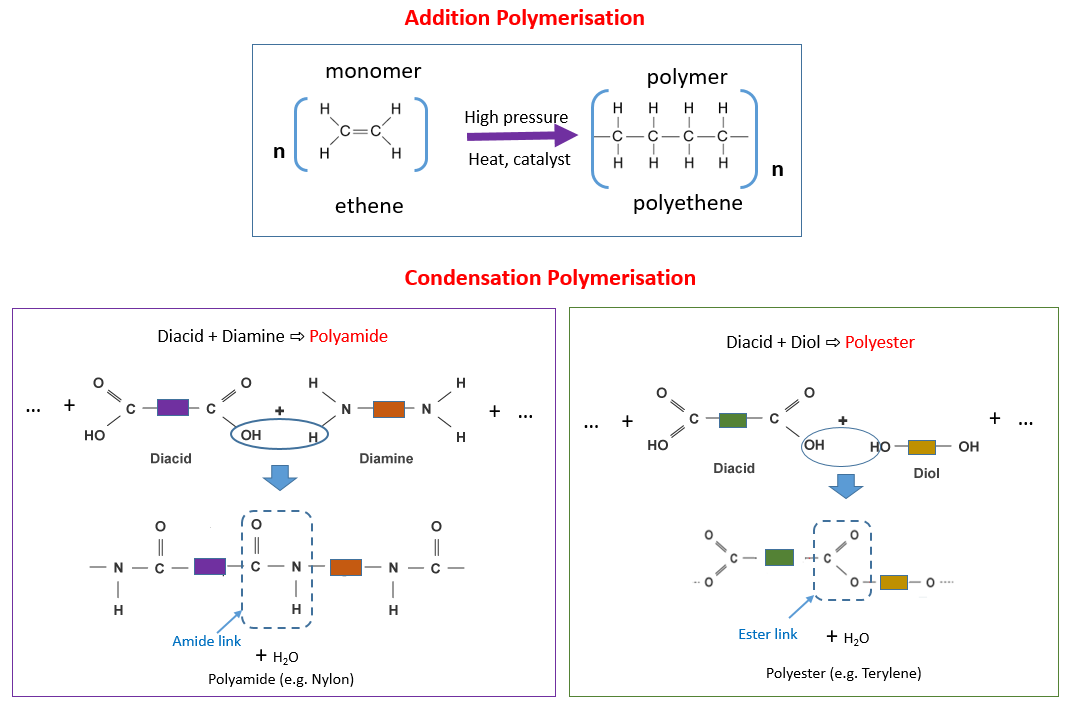
The following diagrams show Addition Polymerisation (Polyethene) and Condensation Polymerisation (Polyamide, Polyester). Scroll down the page for more examples and explanations.
1. Describe what is meant by a condensation polymer.
• Condensation polymers are made from two different monomers.
• Each monomer has two of the same functional groups.
• When these monomers react, small molecules such as water are lost
2. Draw the repeating unit for a condensation polymer.
• Ethane diol has two alcohol groups.
• Hexanedioic has two carboxylic acid groups.
• Ester links are formed and the polymer is called a polyester.
Polymers condensation: Polyamide
A polyamide is formed when a dicarboxylic acid (diacid) combines with a diamine.
To make nylon, we combine hexadioic acid with 1,6 diamino hexane.
A water molecule is removed and the two monomers are joined by the amide link.
Making Nylon: An example of Polyamide
This demonstration shows the production of nylon at the interface between two reactant layers.
A solution of dicarboxylic acid is carefully floated on an aqueous solution of diamone.
Nylon forms at the interface and can be pulled out as fast as it is produced forming a long thread – the ‘nylon rope’.
Procedure:
1. Pour aqueous diamine solution into beaker.
2. Carefully pour the cyclohexane solution of the acid chloride on top of the first solution so that mixing is minimised. Do this by pouring the second solution down the wall of the beaker. The cyclohexane will float on top of the water without mixing.
3. A greyish film of nylon will form at the interface. Pick up a little of this with a pair of tweezers and lift it slowly and gently from the beaker. It should draw up behind it a thread of nylon.
The reaction is a condensation polymerisation
nH2N(CH2)6NH2 + nClOC(CH2)8COCl → H2N[(CH2)6NHCO(CH2)8]nCOCl + nHCl
The nylon formed is nylon 6–10 so called because of the lengths of the carbon chains of the monomers.
The diamine is present in excess to react with the hydrogen chloride that is eliminated. An alternative procedure is to use the stoichiometric quantity of diamine dissolved in excess sodium hydroxide solution
Questions
1. Which small molecule is formed in the condensation reaction in this case?
2. The diamine is present in excess in this method. Why is this useful?
3. Why is the nylon made in this demonstration sometimes referred to as nylon-6,10?
Answers
Formation of the polyester terylene from a dicarboxylic acid (benzene-1,4-dicarboxylic acid) and diol (ethane-1,2-diol).
The process is known as condensation polymerisation - forming water molecules together with polyester molecules. A demonstration to show the formation of a polyester


More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
The following diagrams show Addition Polymerisation (Polyethene) and Condensation Polymerisation (Polyamide, Polyester). Scroll down the page for more examples and explanations.
1. Describe what is meant by a condensation polymer.
• Condensation polymers are made from two different monomers.
• Each monomer has two of the same functional groups.
• When these monomers react, small molecules such as water are lost
2. Draw the repeating unit for a condensation polymer.
• Ethane diol has two alcohol groups.
• Hexanedioic has two carboxylic acid groups.
• Ester links are formed and the polymer is called a polyester.
A polyamide is formed when a dicarboxylic acid (diacid) combines with a diamine.
To make nylon, we combine hexadioic acid with 1,6 diamino hexane.
A water molecule is removed and the two monomers are joined by the amide link.
Making Nylon: An example of Polyamide
This demonstration shows the production of nylon at the interface between two reactant layers.
A solution of dicarboxylic acid is carefully floated on an aqueous solution of diamone.
Nylon forms at the interface and can be pulled out as fast as it is produced forming a long thread – the ‘nylon rope’.
Procedure:
1. Pour aqueous diamine solution into beaker.
2. Carefully pour the cyclohexane solution of the acid chloride on top of the first solution so that mixing is minimised. Do this by pouring the second solution down the wall of the beaker. The cyclohexane will float on top of the water without mixing.
3. A greyish film of nylon will form at the interface. Pick up a little of this with a pair of tweezers and lift it slowly and gently from the beaker. It should draw up behind it a thread of nylon.
The reaction is a condensation polymerisation
nH2N(CH2)6NH2 + nClOC(CH2)8COCl → H2N[(CH2)6NHCO(CH2)8]nCOCl + nHCl
The nylon formed is nylon 6–10 so called because of the lengths of the carbon chains of the monomers.
The diamine is present in excess to react with the hydrogen chloride that is eliminated. An alternative procedure is to use the stoichiometric quantity of diamine dissolved in excess sodium hydroxide solution
Questions
1. Which small molecule is formed in the condensation reaction in this case?
2. The diamine is present in excess in this method. Why is this useful?
3. Why is the nylon made in this demonstration sometimes referred to as nylon-6,10?
Answers
-
Show Solutions
1. Hydrogen chloride, HCl
2. The diamine will react with the hydrochloric acid produced and so it is useful for it to be in excess.
3. This is because the monomers have 6 and 10carbon atoms in their chains respectively.
Formation of the polyester terylene from a dicarboxylic acid (benzene-1,4-dicarboxylic acid) and diol (ethane-1,2-diol).
The process is known as condensation polymerisation - forming water molecules together with polyester molecules. A demonstration to show the formation of a polyester
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.