Group 1: The Alkali Metals
In these lessons, we will learn
- What are alkali metals?
- Physical properties of alkali metals
- Chemical properties of alkali metals
- Reaction with water (watch the videos)
- Reaction with chlorine
- Reaction with oxygen
What are alkali metals?
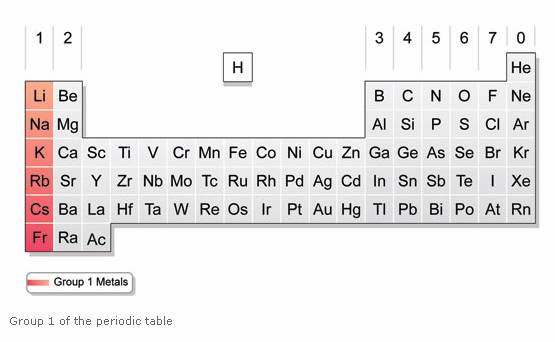
The alkali metals are the six metals of Group 1 in the Periodic Table – lithium, sodium, potassium, rubidium, ceasium (cesium), and francium. Only lithium, sodium and potassium are safe enough to keep in the school lab. Rubidium, ceasium and francium are violently reactive – and francium is radioactive.
The elements in Group 1 are:
Chemical Name |
Chemical Symbol |
Atomic Number |
Lithium |
Li |
3 |
Sodium |
Na |
11 |
Potassium |
K |
19 |
Rubidium |
Rb |
37 |
Caesium |
Cs |
55 |
Francium |
Fr |
87 |
What are the physical properties of alkali metals?
- Good conductors of heat and electricity
- Soft and can be cut by a knife
- Low density (float on water)
- Relatively low melting and boiling points compared to other metals in the Periodic Table
What are the chemical properties of alkali metals?
The alkali metals have 1 valence electron and are the most reactive metals.
How do alkali metals react with water?
- All alkali metals react violently with water, releasing hydrogen gas and forming hydroxides. The hydroxides give alkaline solutions.
Group 1 element + water → alkali + hydrogen
Example:
sodium + water → sodium hydroxide + hydrogen
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Check the videos below to see how each alkali metal reacts with water. You will notice that the alkali metals get more reactive as you go down the group.
Alkali Metal |
How it reacts with water? |
lithium |
a lot of fizz around the floating metal |
sodium |
shoots around on the surface of the water |
potassium |
melts and the hydrogen bursts into flame |
rubidium |
sparks fly |
caesium |
violent explosion |
Why do the alkali metals get more reactive as we go down the group?
This is because as we go down the group because the atoms get larger. This means that the valence electron is further from the nucleus making it easier for the electron to be lost and therefore cause a reaction.
How do Alkali Metals react with Chlorine?
When heated alkali metals are placed into jars of chlorine gas, they will burst into flames. They will burn brightly, giving white solids called chlorides.
Example:
Sodium + chloride → sodium chloride
Na + Cl → NaCl
Sodium chloride is also known as common salt. (It is the salt that we put in our food.) The chloride dissolves in water to give a colorless solution.
How do Alkali Metals react with Oxygen?
When heated alkali metals are placed into jars of oxygen gas, they will burst into flames. They will burn brightly, giving white solids called oxides.
Example
Sodium + oxygen → sodium oxide
4Na + O2 → 2Na 2O
The oxide dissolves in water to give a colorless alkaline solution.
Alkaline Metal |
Color of flame when react with oxygen |
Lithium |
red |
Sodium |
yellow |
Potassium |
lilac |
Alkali metals form ionic compounds
The alkali metals form ionic compounds, in which the metal ion has a charge of 1+. The compounds are white solids, which dissolve in water to give a colorless solution.
Videos of each of the Alkali Metals reacting with water
Lithium
Lithium is the lightest of the alkali metals and very reactive.
The following video shows how lithium reacts with water.
Sodium
Sodium is an alkali metal and element number 11 on the periodic table.
The following video shows how sodium reacts with water.
Potassium
The following video shows how potassium reacts with water. In this video, we see some violent explosions and the gentle creation of a potassium mirror.
Rubidium
The following video shows how rubidium reacts with water. Expect an even bigger explosion.
Caesium or Cesium
The following video shows how cesium reacts with water. But did you know caesium (aka cesium) is also used to define the length of a second?
Francium
Throwing Francium into water would be “the YouTube video of the century” according to the chemistry professor. But is it possible?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.