Balancing chemical equations
Atoms can neither be destroyed nor created during a simple chemical reaction. Therefore, in a chemical reaction the sum of atoms before a reaction is equal to the sum of atoms after a reaction.
The sum of atoms before reaction = the sum of atoms after reaction
This means that chemical equations need to be balanced before they can be used for calculations. There are five common rules (or techniques) that can help us to balance chemical equations. In this lesson, we will elaborate on Rule 4, which is balancing chemical equations using the even/odd technique.
The following figure gives some hints on how to balance chemical equations. Scroll down the page for more examples and solutions.
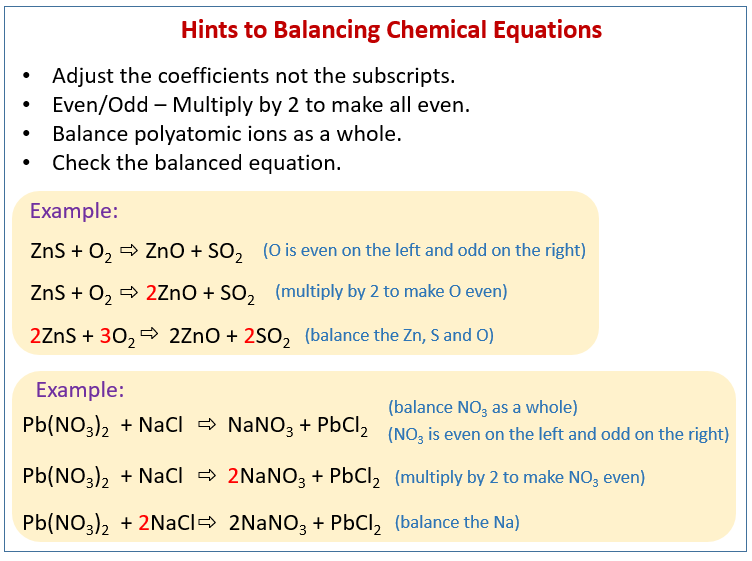
General Rules for balancing chemical equations
| Rule 1Balancing chemical equations using the one’s and two’s technique. | Rule 2Balancing chemical equations using the two’s and three’s technique |
| Rule 3Balancing chemical equations using the CHO technique | Rule 4Balancing chemical equations using the even/odd technique |
| Rule 5Balancing chemical equations containing polyatomic ions |
In this lesson, we will look at some examples of applying
Rule 4: Balancing chemical equations using the even/odd technique.
If you have an even number of a certain element on one side of the equation and an odd number of the same element on the other side of the equation, multiply both sides of the equation through by the coefficient of 2. This will give an even number on both sides and make the equation easier to balance.
Example:
Balance the equation
CH3OH + O2 → H2O + CO2
Solution:
Step 1: Using the CHO technique, we start with carbon, one on each side, so carbon is balanced. There are four H on the left and two on the right, so we place the coefficient of 2 in front of the H2O on the right
CH3OH + O2 → 2H2O + CO2
Step 2: When we try to balance the oxygen, we find three on the left and four on the right. We multiply both sides of the equation through by two.
2CH3OH + 2O2 → 4H2O + 2CO2
Step 3: The C and H are still balanced, and now there are six O on the left and eight on the right. Change the coefficient in front of the O2 to 3 to give eight O on the left.
2CH3OH + 3O2 → 4H2O + 2CO2
Step 4: Check that all the atoms balance and make sure that all coefficients are in the lowest-possible ratio.
Balancing Equations Even Odd #1
Balancing Equations Even Odd #2
Balancing Challenging Chemical Equations
How to balance a type of challenging chemical equation called the “Odd-Even” types.
Balancing chemical equations can be challenging.
This video shows how to use the T-chart and the even/odd technique.
A Beginner’s Guide to Balancing Equations
This video explains the basics of balancing chemical equations. A visual guide shows you how to change coefficients to balance the atoms in reactants and products.
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.