Chemical Analysis
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
Chemical analysis and investigation, Inorganic analysis, Organic analysis, Experimental design, and investigation, Practical examinations.
Tests for anions, cations, carbonate ions, halide ions, nitrates ions, sulfate ions, sulfide ions, ammonia, carbon dioxide, chlorine, hydrogen, oxygen, sulfur dioxide, flame tests for metal ions, test for water, acid, base, alkane, alkene and unsaturated hydrocarbons.
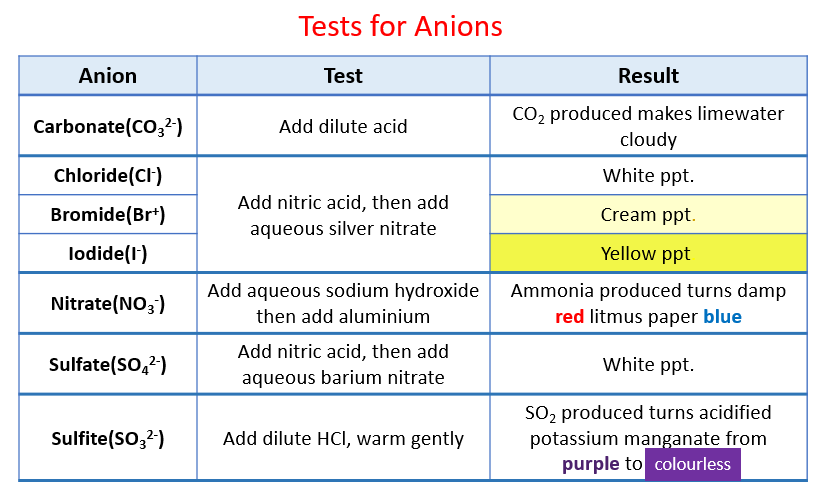
The following table shows the tests for anions:
Carbonate, Chloride, Bromide, Iodide, Nitrate, Sulfate and Sulfite. Scroll down the page for examples of how to use the Anion Tests.
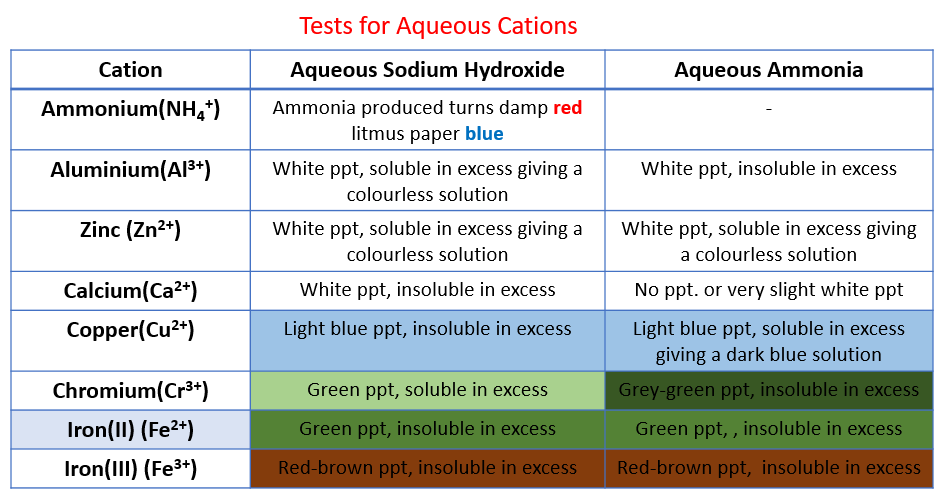
The following table shows the tests for cations:
Aluminium, ammonium, calcium, chromium(III), copper, iron(II), iron(III), Zinc. Scroll down the page for examples of how to use the Cation Tests.
Testing for carbonate ions in solution.
Add dilute acid effervescence, carbon dioxide produced
Testing for halide ions in solution: Chloride, Bromide, Iodide
Acidify with dilute nitric acid, then add aqueous silver nitrate
Chloride - white ppt, Bromide - cream ppt, Iodide - yellow ppt.
Testing for nitrate ions in solution
Add aqueous sodium hydroxide, then aluminium foil; warm carefully, ammonia produced
Testing for sulfate ions in solution
Acidify, then add aqueous barium nitrate, white ppt formed.
Testing for sulfide ions in solution
Add dilute hydrochloric acid, warm gently and test for the presence of sulfur dioxide. Sulfur dioxide produced will turn acidified aqueous potassium manganate(VII) from purple to colourless
Testing for Cations
Copper, Iron(II), Iron(III), Zinc, Ammonium
The following table shows the tests for gases:
Ammonia, Carbon Dioxide, Chlorine, Hydrogen, Oxygen, Sulfur Dioxide. 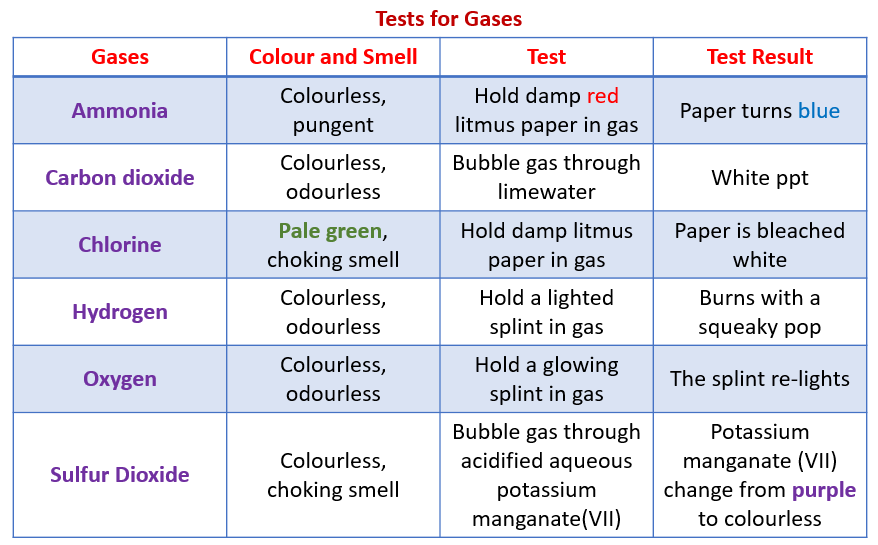
The Tests for Carbon Dioxide, Oxygen and Hydrogen
carbon dioxide (limewater goes milky); oxygen (a glowing splint re-lights) and hydrogen (a lighted splint produces a ‘squeaky pop’ sound).
The following table shows the flame tests for metal ions:
Lithium - Red, Sodium - Yellow, Potassium - Lilac, Copper(II) - blue-green. 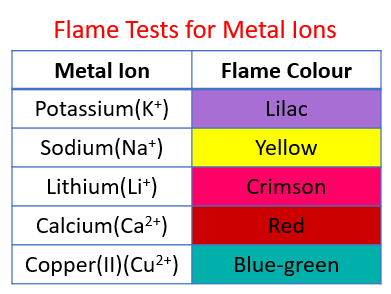
Flame Test of Ions Lab Experiment - Li, Na, K, Sr, and Cu Ions
The following table shows the tests for water, acid, base, alkane and alkene.

Test for Unsaturated Hydrocarbons
The bromine water is initially an orange-brown colour. If the liquid being tested turns the bromine colourless then the compound id unsaturated - it contains at least one double bond. Alkanes are saturated and would not react.
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.