Heating and Cooling Graphs
A series of free GCSE/IGCSE Physics Notes and Lessons.
The following diagrams show a heating curve and a cooling curve. Scroll down the page for more examples and solutions.
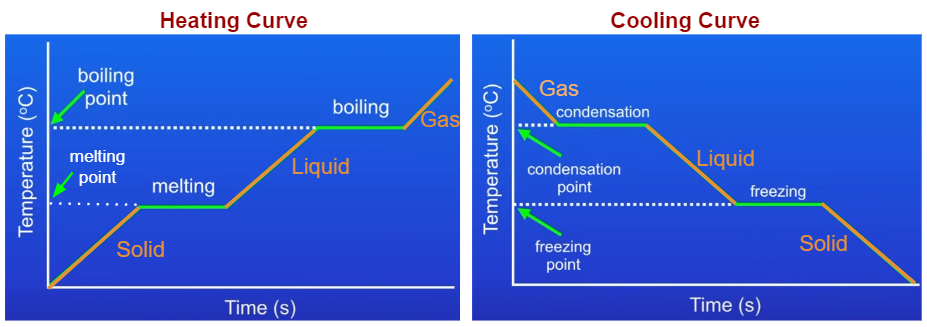
Heating Curve
- The temperature of the solid rises as we increase the energy of the particles.
- At the melting point, the temperature stops rising and the line becomes horizontal.
- The solid continues to melt. The energy we put in is weakening or breaking the forces of attraction between the particles, allowing the substance to change from a solid to a liquid. The energy is called the latent heat. There is a change of state but no change of temperature.
- When all the solid has melted, the temperature of the liquid will rise again.
- At the boiling point, the temperature stops rising again and the line becomes horizontal.
- When all of the liquid has boiled, the temperature of the gas will rise again.
Cooling Curve
- The temperature of the gas decreases as we decrease the energy of the particles.
- At the condensation point, the temperature stops dropping and the line becomes horizontal.
- The gas continues to condense. There is a change of state from gas to liquid but no change of temperature.
- When all the gas has condensed, the temperature of the liquid will drop again.
- At the freezing point, the temperature stops dropping again and the line becomes horizontal.
- When all of the liquid is frozen, the temperature of the solid will drop again.
Heating and Cooling Graphs
Interpret heating and cooling graphs that include change of state.
What happens to substances when we heat them and the changes of state from graphs?
Reading Heating and Cooling Curves
How to read a heating or cooling curve?
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.