Properties of Metals
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Lessons (Cambridge IGCSE Chemistry).
Patterns and properties of metals, The alkali metals, Aluminium, The transition elements, The reactivity of metals, Electrical cells and energy.
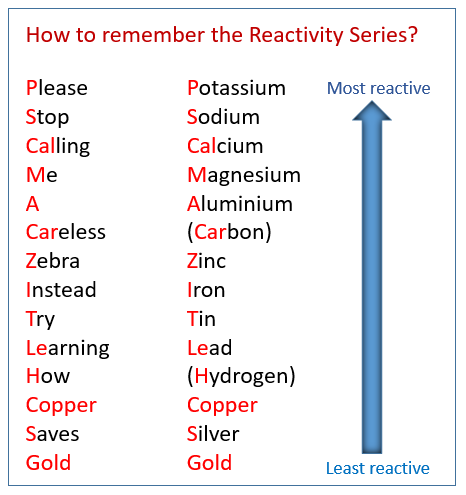
The following table shows the reactivity series of metals and how to remember them using a mnemonic. Scroll down the page for examples and solutions.
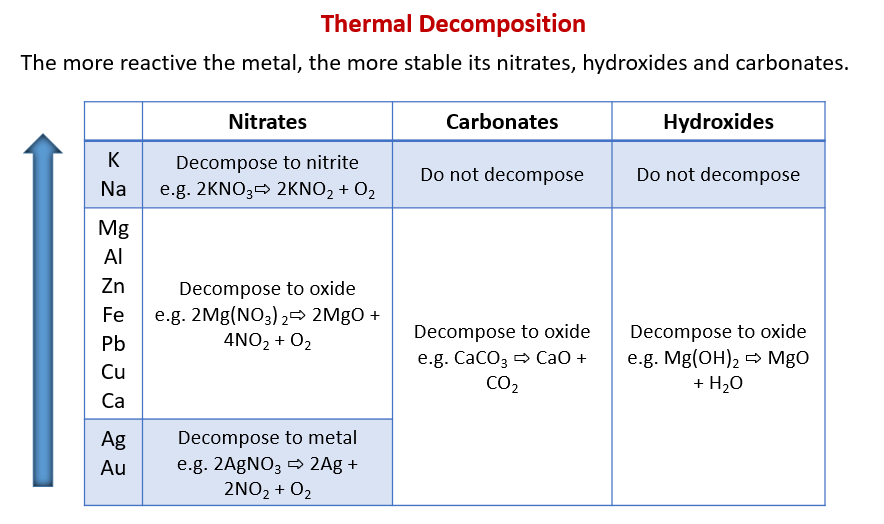
The following table shows the Thermal Decomposition for metal nitrates, carbonates and hydroxides. Scroll down the page for examples and solutions.
Reactions of Alkaline Earth Metals with Oxygen
How Group II metals burn in oxygen?
Reactions of Alkaline Earth Metals with Water
What Are The Uses Of Aluminium?
How Does It Anodise?
Thermite Reaction
iron(III) oxide(s) + aluminium → aluminium oxide + iron
Test for Aluminium ions
Solutions containing aluminium ions are typically colourless in colour.
To test for the presence of aluminium ions in solution, you may use either the sodium hydroxide test or the ammonia test.
- Sodium Hydroxide Test - To a sample of your solution, add a few drops of sodium hydroxide solution. A white precipitate forms. Upon addition of excess sodium hydroxide, the white precipitate dissolves.
- Ammonia Test - To a sample of your solution, add a few drops of ammonia solution. A white precipitate forms. Upon addition of excess ammonia solution, the white precipitate remains insoluble.
Testing cations
Ammonium, Copper II, Iron II, Iron III, Zinc
Displacement reactions
Thermal decomposition of metal compounds 1/2
Thermal decomposition of metal compounds 2/2
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.