

Molarity
Related Topics:
More Lessons for Chemistry
Math Worksheets
This is a series of lectures in videos covering Chemistry topics taught in High Schools.
What is Molarity?
How calculate Molarity or calculating Molarity and Molar Concentration?
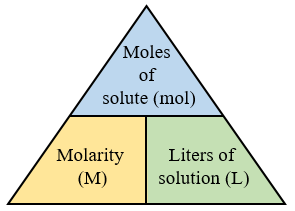
The following diagram shows how to convert between Molarity, Moles and Volume. Scroll down the page for more examples and solutions.
Concentration, Solutes, Solvents, and Molarity
Example:
What is the molarity of the solution with 0.70 mol solute in 250 mL solution?
Molarity
The concept of molarity is explained and problems determining molarity are solved.
Example:
1. Calculate the molarity of a solution made by dissolving 5.4 g NaCl in 25 mL of solution.
2. Calculate the molarity of a solution made by dissolving 10.3 g sodium sulfate in 600 mL of solution. What is the concentration of the Na+
3. How many grams of NaOH are needed to make 400 mL of a 1.5 M solution?
Molarity/Molar Concentrations
How to calculate Molarity/Molar Concentration?
Molar concentration is the number of moles of solute that can dissolve in 1L of solution.
Molar concentration is often referred to as molarity.
Example:
1. A saline solution contains 0.9g of sodium chloride, NaCl, dissolved in 100mL of solution. What is the molar concentration of the solution?
2. At 20°C, a saturated solution of calcium sulfate, CaSO4, has a concentration of 0.0153 mol/L. A student takes 65mL of this solution and evaporates it. What mass (in g) is left in the evaporating dish?
Molarity
Examples:
1. What is the molarity of a solution that contains 6 mols of solute in 2 liters of solution?
2. How many moles are in 600mL of a 1.5M solution of CaCO3?
How to Calculate Molarity and make Solutions?
Molarity is defined as moles of solute per liters of solution.
The formula is:
M = n / V
M is molarity
n is number of moles of solute
V is volume of the solution in liters
Example:
1. What is the molarity of a 125 mL solution containing 0.050 moles of hydrochloric acid?
2. How many moles of sodium hydroxide are in 38 mL of 0.50 mol/L NaOH?
3. How much 25 mol/L sulfuric acid should you use if you need 0,12 mol H2SO4?
4. How would you make 100 mL of 0.40 M of copper(II) sulfate? Molarity


More Lessons for Chemistry
Math Worksheets
This is a series of lectures in videos covering Chemistry topics taught in High Schools.
What is Molarity?
How calculate Molarity or calculating Molarity and Molar Concentration?
The following diagram shows how to convert between Molarity, Moles and Volume. Scroll down the page for more examples and solutions.
Concentration, Solutes, Solvents, and Molarity
Example:
What is the molarity of the solution with 0.70 mol solute in 250 mL solution?
Molarity
The concept of molarity is explained and problems determining molarity are solved.
Example:
1. Calculate the molarity of a solution made by dissolving 5.4 g NaCl in 25 mL of solution.
2. Calculate the molarity of a solution made by dissolving 10.3 g sodium sulfate in 600 mL of solution. What is the concentration of the Na+
3. How many grams of NaOH are needed to make 400 mL of a 1.5 M solution?
Molarity/Molar Concentrations
How to calculate Molarity/Molar Concentration?
Molar concentration is the number of moles of solute that can dissolve in 1L of solution.
Molar concentration is often referred to as molarity.
Example:
1. A saline solution contains 0.9g of sodium chloride, NaCl, dissolved in 100mL of solution. What is the molar concentration of the solution?
2. At 20°C, a saturated solution of calcium sulfate, CaSO4, has a concentration of 0.0153 mol/L. A student takes 65mL of this solution and evaporates it. What mass (in g) is left in the evaporating dish?
Examples:
1. What is the molarity of a solution that contains 6 mols of solute in 2 liters of solution?
2. How many moles are in 600mL of a 1.5M solution of CaCO3?
How to Calculate Molarity and make Solutions?
Molarity is defined as moles of solute per liters of solution.
The formula is:
M = n / V
M is molarity
n is number of moles of solute
V is volume of the solution in liters
Example:
1. What is the molarity of a 125 mL solution containing 0.050 moles of hydrochloric acid?
2. How many moles of sodium hydroxide are in 38 mL of 0.50 mol/L NaOH?
3. How much 25 mol/L sulfuric acid should you use if you need 0,12 mol H2SO4?
4. How would you make 100 mL of 0.40 M of copper(II) sulfate? Molarity
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.