Naming Alkenes and Alcohols
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Naming Alkenes
ethene, propene, 1-butene, 2-butene, 3-butene, cyclic alkene 4,6-dimethyl-2-haptene
Cyclic Hydrocarbons
A cyclic hydrocarbon consists of carbon and hydrogen atoms where the carbon atoms are arranged in the form of a ring. A cycloalkane is a cyclic hydrocarbon that contains only carbon - carbon single bonds. A cycloalkene and cycloalkyne are cyclic hydrocarbons that contain carbon - carbon double and triple bonds respectively.
The generic formula for cycloalkanes is CnH2n, which is the same generic formula as alkenes. The cycloalkanes have the molecular formula of alkenes but was saturated.
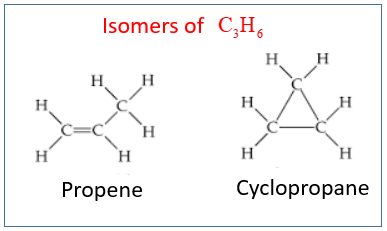
The following diagram shows the isomers for C3H6: Propene and Cyclopropane.
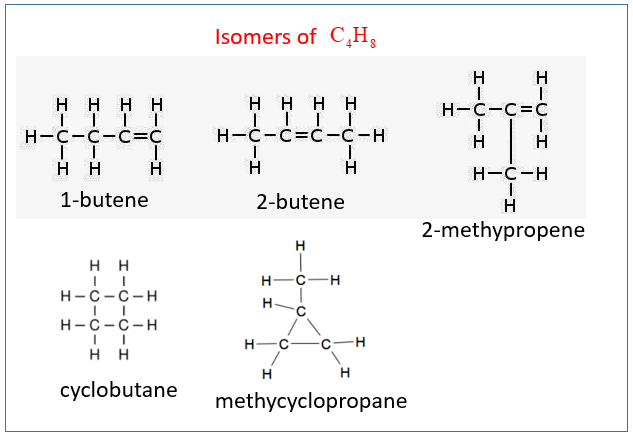
1. Draw and name the isomers of C4H8. Remember to include the cyclic hydrocarbons
Answers
Alcohol
Draw the structures of methanol, ethanol and propanol.
Describe the reactions of these alcohols.
• Methanol, ethanol and propanol all dissolve in water to form a neutral solution.
• They react with sodium to produce hydrogen gas.
• They are used as solvents and fuels. Ethanol is found in alcoholic drinks.
• Alcohols burn in air to produce carbon dioxide and water.
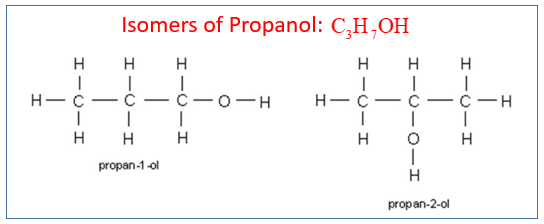
Describe how ethanol can be oxidised to ethanoic acid. Position isomers of propanol Questions
1. Draw and name two isomers that are alcohols with the formula C3H7OH. Here it is simply the position of the functional group in the chain that has changed. It is called position isomerism.
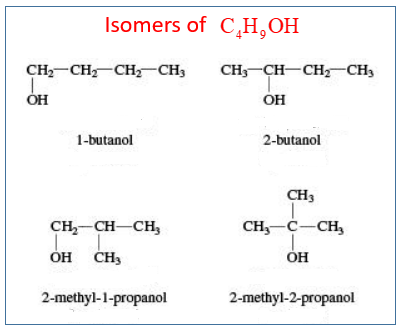
2. Draw and name the isomers that are alcohols with the formula C4H9OH?
Answers


More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Naming Alkenes
ethene, propene, 1-butene, 2-butene, 3-butene, cyclic alkene 4,6-dimethyl-2-haptene
A cyclic hydrocarbon consists of carbon and hydrogen atoms where the carbon atoms are arranged in the form of a ring. A cycloalkane is a cyclic hydrocarbon that contains only carbon - carbon single bonds. A cycloalkene and cycloalkyne are cyclic hydrocarbons that contain carbon - carbon double and triple bonds respectively.
The generic formula for cycloalkanes is CnH2n, which is the same generic formula as alkenes. The cycloalkanes have the molecular formula of alkenes but was saturated.
The following diagram shows the isomers for C3H6: Propene and Cyclopropane.
1. Draw and name the isomers of C4H8. Remember to include the cyclic hydrocarbons
Answers
Draw the structures of methanol, ethanol and propanol.
Describe the reactions of these alcohols.
• Methanol, ethanol and propanol all dissolve in water to form a neutral solution.
• They react with sodium to produce hydrogen gas.
• They are used as solvents and fuels. Ethanol is found in alcoholic drinks.
• Alcohols burn in air to produce carbon dioxide and water.
Describe how ethanol can be oxidised to ethanoic acid. Position isomers of propanol Questions
1. Draw and name two isomers that are alcohols with the formula C3H7OH. Here it is simply the position of the functional group in the chain that has changed. It is called position isomerism.
2. Draw and name the isomers that are alcohols with the formula C4H9OH?
Answers
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.