

Redox Reactions
Related Topics:
More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Redox Reactions
The following table shows the Redox Reaction. Scroll down the page for examples and solutions.
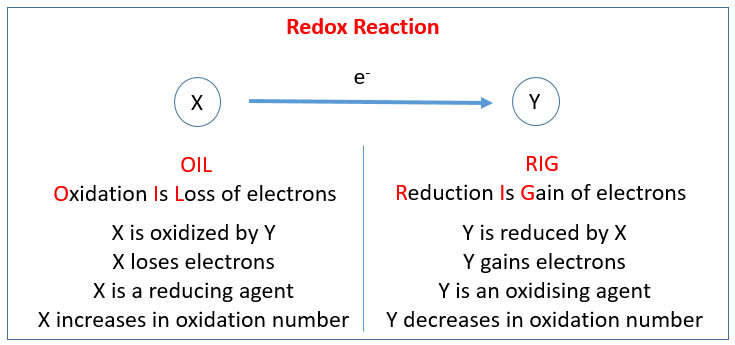
An oxidation reduction (redox) reaction happens when electrons are transferred between atoms.
A loss of electrons is called oxidation, and we say that atom has become oxidized.
A gain of electrons is called reduction, and we say that the atoms has become reduced.
The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction.
Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
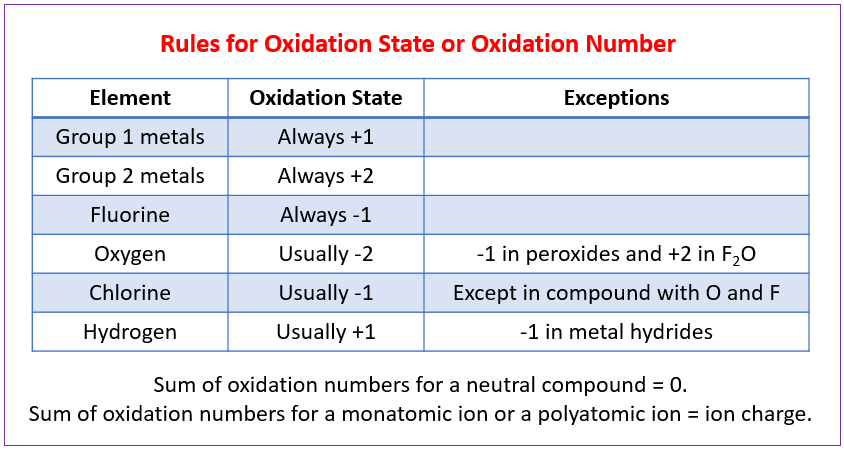
How to Calculate Oxidation Numbers (Oxidation State)?
How to determine the oxidation numbers or oxidation states for the elements in a chemical compound?
The oxidation numbers tell us how electrons are divided up or shared between atoms in a chemical compound.
The oxidation numbers also tell us how electrons move in an oxidation reduction (redox) reaction.
Oxidizing Agents and Reducing Agents
Oxidizing agents make oxidation happen, and reducing agents make reduction happen.
An oxidizing agent takes electrons from something, allowing it to be oxidized, and a reducing agents gives electrons to something, allowing it to be reduced.
The thing that is reduced is the oxidizing agent, and the thing that is oxidized is the reducing agent.
In order to identify the oxidizing and reducing agents, we have to write oxidation numbers (or oxidation states) for the elements in the equation, and then figure out how electrons are moving, what is being oxidized and what is being reduced. Test for Oxidising Agents
Method:
1. Add potassium iodide solution to the unknown liquid.
2. If an oxidising agent is present, a red-brown colour appears.
(The iodide ion in the potassium iodide solution is oxidised to iodine)
Test for Reducing Agents
Method:
1. Add the unknown liquid to the potassium manganate(IV) solution.
2. If a reducing agent is present, the purple colour fades.
The Mn7+ is reduced to Mn2+ resulting in the colour change.


More Lessons for IGCSE Chemistry
Math Worksheets
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry).
Redox Reactions
The following table shows the Redox Reaction. Scroll down the page for examples and solutions.
An oxidation reduction (redox) reaction happens when electrons are transferred between atoms.
A loss of electrons is called oxidation, and we say that atom has become oxidized.
A gain of electrons is called reduction, and we say that the atoms has become reduced.
The two separate parts (oxidation and reduction) of an oxidation reduction (redox) reaction are called half reactions. Two half reactions can be put together to make the whole reaction.
Oxidation numbers are numbers that can be written above atoms to show whether they are gaining or losing electrons.
How to determine the oxidation numbers or oxidation states for the elements in a chemical compound?
The oxidation numbers tell us how electrons are divided up or shared between atoms in a chemical compound.
The oxidation numbers also tell us how electrons move in an oxidation reduction (redox) reaction.
Oxidizing Agents and Reducing Agents
Oxidizing agents make oxidation happen, and reducing agents make reduction happen.
An oxidizing agent takes electrons from something, allowing it to be oxidized, and a reducing agents gives electrons to something, allowing it to be reduced.
The thing that is reduced is the oxidizing agent, and the thing that is oxidized is the reducing agent.
In order to identify the oxidizing and reducing agents, we have to write oxidation numbers (or oxidation states) for the elements in the equation, and then figure out how electrons are moving, what is being oxidized and what is being reduced. Test for Oxidising Agents
Method:
1. Add potassium iodide solution to the unknown liquid.
2. If an oxidising agent is present, a red-brown colour appears.
(The iodide ion in the potassium iodide solution is oxidised to iodine)
Test for Reducing Agents
Method:
1. Add the unknown liquid to the potassium manganate(IV) solution.
2. If a reducing agent is present, the purple colour fades.
The Mn7+ is reduced to Mn2+ resulting in the colour change.
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.



We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.