Isotopes & Relative Atomic Mass
Related Pages
Mole Calculation
Molecular Mass
More Lessons on Chemistry
In these lessons, we will learn about Isotopes, Isotope Notation, Atomic Mass Unit (amu), and how to calculate the Atomic Mass of an element.
Isotopes
Isotopes are atoms of the same chemical element that have the same number of protons but different numbers of neutrons.
Because they have the same number of protons, isotopes belong to the same element and therefore have nearly identical chemical properties. The difference in the number of neutrons means they have different mass numbers, which is the total number of protons and neutrons in the nucleus.
The following diagrams show the isotopes of chlorine and how to calculate the relative atomic mass. Scroll down the page for examples and solutions.
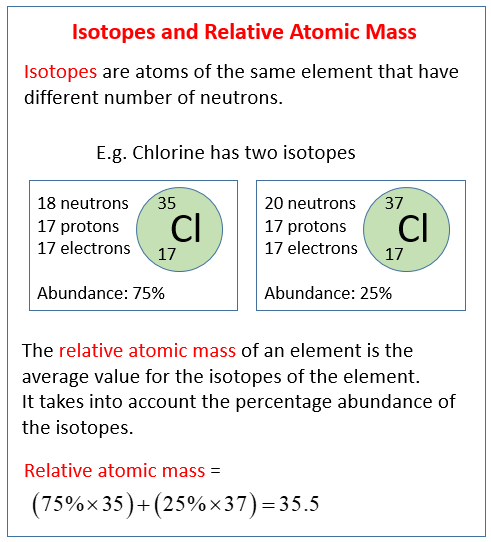
Relative Atomic Mass (Ar or RAM)
The relative atomic mass (Ar) of an element is the weighted average of the masses of its naturally occurring isotopes, relative to 1/12th the mass of a carbon-12 atom.
Most elements found in nature are a mixture of several isotopes. The periodic table lists a single atomic mass for each element, and this value is not simply the mass of the most common isotope. Instead, it’s an average that takes into account the mass of each isotope and its relative abundance (how common it is) in a typical sample of that element.
Relative atomic mass is a dimensionless quantity (it has no units) because it is a ratio of two masses. It tells you how many times heavier an average atom of an element is compared to 1/12th the mass of a carbon-12 atom (which is defined as exactly 12 atomic mass units, or amu).
Examples:
\(^{35}_{17}Cl\) is an isotope of chlorine that has 17 protons and 18 neutrons.
\({^{37}_{17}Cl}\) is an isotope of chlorine that has 17 protons and 20 neutrons.
Generally, isotopes behave the same way during chemical reactions. The extra neutrons just change the mass of the atom and its density.
Some of the atoms of certain isotopes are unstable because of the extra number of neutrons, and they are said to be radioactive.
Calculation of Relative Atomic Mass:
To calculate the relative atomic mass of an element, you need:
The mass of each isotope.
The percentage abundance of each isotope.
The formula is
\(A_{r}=\frac{\text{Sum of (Isotope Mass}\times \text{Abundance)}}{100}\)
Videos
What are Isotopes?
In this video, we’ll learn about what isotopes are and how to write atomic number and mass number
in isotope notation. We talk about a simple analogy with cars to explain this tutorial.
Isotopes are versions of an atom or an element that have the same number of protons, but different numbers of neutrons. Isotopes and isotope notation are particularly important in nuclear chemistry.
Isotope Notation
Learn how to write atoms in isotope notation! In isotope notation, you can quickly show how many
protons, neutrons, and electrons are in an atom. You put the atomic number, mass number, and net
charge around the chemical element symbol.
Isotope notation is particularly important in nuclear chemistry, because if you’re doing fission, fusion, alpha decay, beta decay, positron emission, or electron capture, you want to be able to tell how many neutrons and protons are in the nucleus.
Standard atom - Atomic Mass Unit
Since the mass of an atom would be extremely small when measured in grams, it would be more convenient to measure the masses of atoms relative to a standard atom.
The standard atom chosen is \(\ce{^{12}_{6}C}\) (carbon-12) isotope. An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu).
Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron)
= \(\frac{1}{12} \times \) mass of one carbon-12 atom
= 1 amu (atomic mass unit)
Atomic Mass Units
Average Atomic Mass and definition of atomic mass unit
Relative atomic mass
The relative atomic mass (Ar) of an element is the average mass of the naturally occurring atoms of the element. This quantity takes into account the percentage abundance of all the isotopes of an element which exist.
The formula for relative atomic mass is
Ar = average mass of isotopes of the element
Example:
Given that the percentage abundance of ![]() is 75% and that of
is 75% and that of ![]() is 25%, calculate the Ar of chlorine.
is 25%, calculate the Ar of chlorine.
Solution:
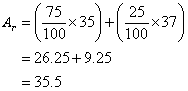
Example:
Bromine has two isotopes, Br-79 and Br-81. Both exist in equal amounts. Calculate the relative
atomic mass of bromine.
Solution:
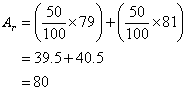
Example:
The neon element has three isotopes. They are 90.92% of ![]() , 0.26% of
, 0.26% of ![]() and 8.82% of
and 8.82% of ![]()
Solution:
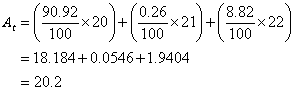
Atomic Mass: Introduction
What is atomic mass? It is a weighed average of the different isotopes of an element. It is sometimes referred to as atomic weight, relative atomic mass, or average atomic mass. We look at how to calculate and determine the weighed average of elements using atomic mass units.
Atomic Mass: How to Calculate Isotope Abundance
How do you determine and calculate isotope abundance when you know the relative atomic mass (also known as atomic weight), as measured in amu or atomic mass numbers? Here we will go through the algebra and reasoning to figure out the amount of abundances of the isotopes, in percentages and in decimals.
Example:
There are two stable isotopes of chlorine: Chlorine-35 (which weighs 34.97 amu) and
Chlorine-37 (which weighs 36.97 amu). If the relative atomic mass of chlorine is 35.45,
what is the abundance of each isotope?
Try out our new and fun Fraction Concoction Game.
Add and subtract fractions to make exciting fraction concoctions following a recipe. There are four levels of difficulty: Easy, medium, hard and insane. Practice the basics of fraction addition and subtraction or challenge yourself with the insane level.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.