Specific Heat Capacity Experiment
Related Pages
Specific Heat Capacity
Energy Transfers
Mechanical, Potential and Kinetic Energy
Elastic Potential Energy
Lessons for IGCSE Physics
A series of free GCSE/IGCSE Physics Notes and Lessons.
In these lessons, we will learn to describe a practical that can be used to determine the specific heat capacity of a material.
Specific Heat Capacity Experiment
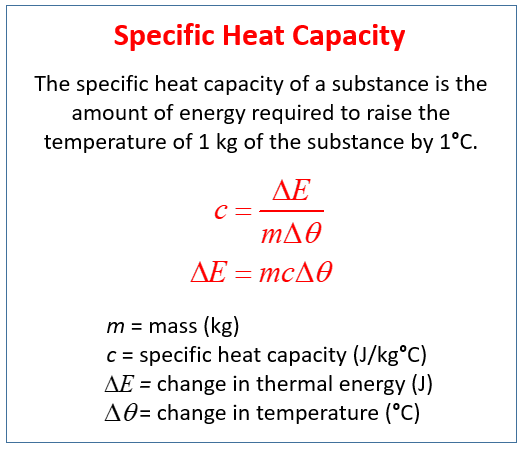
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1 kg of the substance by 1°C.
Specific Heat Capacity Practical
Steps to determine the specific heat capacity.
- Place a beaker on a balance and press zero.
- Now add the oil to the beaker and record the mass of the oil.
- Read the starting temperature of the oil.
- Connect a joulemeter to the immersion heater.
- Time for thirty minutes.
- Read the number of joules of energy that passed into the immersion heater.
- Read the final temperature of the oil.
- Use the following formula to calculate the specific heat capacity.
Results and Calculations
0.95 kg of oil was heated from 20°C to 75 °C. 87258J of electrical energy passed into the immersion heater. Calculate the specific heat capacity of the oil.
Sources of inaccuracies
- Thermal energy passing out of the beaker into the air - Use an insulator with a lower thermal conductivity.
- Not all thermal energy passing into the oil - Ensure that immersion heater is fully submerged.
- Incorrect reading of thermometer - Use an electronic temperature probe.
- Thermal energy not being spread through the oil - Stir the oil.
Experiment to show how to find the specific heat capacity of a metal
Specific Heat Capacity - GCSE Science Required Practical
Investigating the specific heat capacity of different metals.
In this practical you will:
- heat up blocks of different metals using an electric heater
- measure the mass and temperature of the block
- calculate the work done by the heater
- plot a graph of temperature change against work done and use the gradient to calculate the specific heat capacity of the metal
Method
- Measure and record the mass of the copper block in kg.
- Wrap the insulation around the block.
- Place the heater in the larger hole in the block.
- Connect the ammeter, power pack and heater in series.
- Connect the voltmeter across the heater.
- Use the pipette to put a small amount of water in the other hole.
- Put the thermometer in this hole.
- Set the power pack to 12 V. Switch on the power pack to turn on the heater.
- Record the ammeter and voltmeter readings. These shouldn’t change during the experiment.
- Measure the temperature and start the stopclock.
- Record the temperature every minute for 10 minutes.
Record your results in the table below. - Calculate the power of the heater in watts.
Power in watts = potential difference in volts x current in amps - Calculate the energy transferred (work done) by the heater. To do this, multiply the time in seconds by the power of the heater. Record these values in your table.
Specific Heat Capacity - GCSE Science Required Practical
How to measure the SHC of a material using the graph method?
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.