Stoichiometry - Limiting and Excess Reactant
A series of free IGCSE Chemistry Activities and Experiments (Cambridge IGCSE Chemistry). In these lessons we look at the limiting reactant in a chemical reaction
Related Pages
Stoichiometry Lessons
Molar Volume
More Lessons for IGCSE Chemistry
More Science Lessons (KS3/Checkpoint 1)
More Science Lessons (KS3/Checkpoint 2)
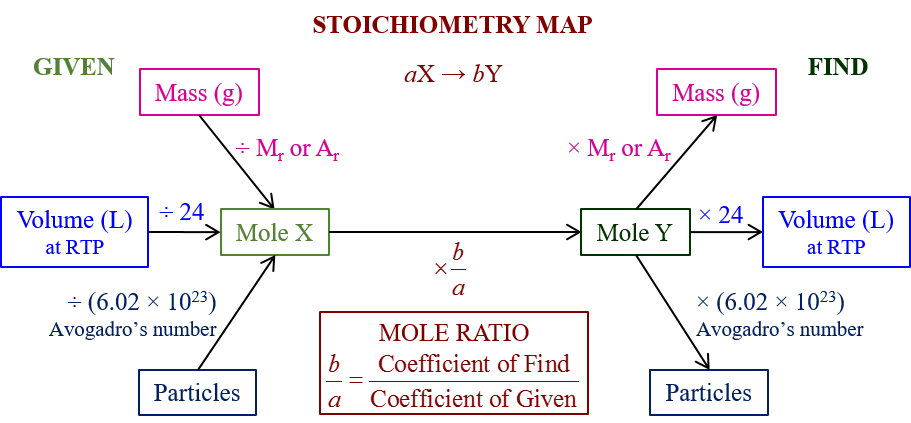
The following Stoichiometry Road Map gives a summary of how to use stoichiometry to calculate moles, masses, volumes and particles in a chemical reaction with limiting and excess reactants. Scroll down the page for more examples and solutions.
Stoichiometry - Limiting and Excess Reactant
Introduction to Limiting Reactant and Excess Reactant
The limiting reactant or limiting reagent is the first reactant to get used up in a chemical reaction.
Once the limiting reactant gets used up, the reaction has to stop and cannot continue and there is extra of the other reactants left over. Those are called the excess reactants.
We will learn about limiting reactant and limiting reagent by comparing chemical reactions to cooking recipes and we will look at an actual stoichiometry problem.
Example:
What is the greatest amount of NH3 (in moles) that can be made with 3.2 moles of N2 and 5,4 moles of H2? What is the limiting reactant? Which reactant is in excess and how many moles of it are left over?
Limiting Reactant Practice Problem (moles)
To solve stoichiometry problems with limiting reactant or limiting reagent:
- Figure out which of the reactants is the limiting reactant or limiting reagent.
- See how much product can be formed by using the maximum amount of the limiting reactant or limiting reagent.
- The excess reactant is what is left over after all of the limiting reactant has been used up.
Example:
- What is the greatest amount of MgO (in moles) that can be made with 7.8 moles of Mg and 4.7 moles of O2? Which is the limiting reactant? Which reactant is in excess and how many moles of it are left over?
Limiting Reactant Problem (grams)
Example:
- What is the greatest amount of AlCl3 (in grams) that can be made with 114 grams of Al and 186 grams of Cl2? Which is the limiting reactant? Which reactant is in excess, and how many grams of it are left over?
Questions:
-
Take the reaction:
NH3 + O2 → NO + H2O.
In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2.
a. Which reactant is the limiting reagent?
b. How many grams of NO are formed?
c. How much of the excess reactant remains after the reaction? -
If 4.95 g of ethylene (C2H4) are combusted with 3.25 g of oxygen.
a. What is the limiting reagent?
b. How many grams of CO2 are formed? -
A reaction container holds 5.77 g of P4 and 5.77 g of O2.
The following reaction occurs: P4 + O2 → P4O6.
If enough oxygen is available then the P4O6 reacts further: P4O6 + O2 → P4O10.
a. What is the limiting reagent for the formation of P4O10?
b. What mass of P4O10 is produced?
c. What mass of excess reactant is left in the reaction container?
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.